
Meet us on CPhI in Milan.
Chiracon GmbH will also be exhibiting at the convention for pharmaceutical ingredients in Milan from 8-10 October 2024.
We look forward to welcoming you at our booth B17 in hall 4, or you are welcome to make an appointment with us in advance. Contact us – Chiracon GmbH

With Chiracon to new entities and APIs
Chiracon GmbH was founded in 1998 with the aim of becoming a global partner for the production of high-quality intermediates and end products for the pharmaceutical sector using innovative manufacturing processes. We offer first-class and reliable service in the areas of contract research, intermediates, scale-up, active pharmaceutical ingredients (API), stability studies and clinical trial samples.
Benefit from our product portfolio
We assume project responsibility for the development and optimisation of synthesis routes and manufacturing processes, including quality assurance, stability testing and documentation for approval.
Our approach is modular and is customised to your needs in close collaboration, so that we accompany you from the structure to the desired product.
APIs in GMP-quality for the pharmaceutical industry and large pharmacies.

Synthesis development for products from the mg to the kg range.
Scaling from laboratory to production scale.
Especially chiral intermediates for synthesis and technical applications

Highly standardised stability studies according to ICH guidelines and customer-specific requirements

Production of APIs for clinical trials, including the associated documentation
We are building the European future of API production in Brandenburg
A state-of-the-art production and office building is being built in the immediate vicinity of the Biotechnology Park in Luckenwalde. This will enable us to fulfil the requirements of our customers and the authorities even better than today. The new building and the production facilities will of course also fulfil the highest environmental standards.

We are looking for you!
We are regularly looking for motivated and committed employees in the areas of administration, laboratory, production, research, development and quality assurance. Chemists, biochemists, chemical laboratory assistants, biology laboratory assistants and commercial staff are an essential part of our team.
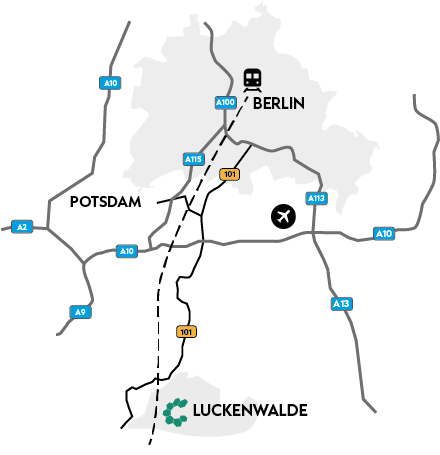
We are closer than you think, only 45 minutes by train from Berlin Central Station or 40 minutes by car from BER Airport. You will find us in the innovative Luckenwalde Biotechnology Park, surrounded by the green Brandenburg countryside.
News of Chiracon

Ceremonial laying of the foundation stone
On 13 June 2024, the foundation stone was ceremoniously laid on the construction site of the new building for Chiracon GmbH in Luckenwalde near the biotechnology park. After speeches by State Secretary Hendrik Fischer, architect Jörg Vogt and CEO of Chiracon GmbH Dr. Ralf Zuhse, a time capsule was ceremoniously walled in. The ceremony was […]

Chiracon on Chemspec in Düsseldorf
Chiracon GmbH will be exhibiting at the 37th International Exhibition for Fine and Speciality Chemicals in Düsseldorf from 19 – 20 June 2024. We look forward to seeing you at our booth J50.

The first wall of the new building is up
Following the start of the construction project, the construction site for the new state-of-the-art production facility with administration building for Chiracon is making great strides forward. With the completion of work on the foundations of the administration building, work began on the first walls. The first part of the outer wall is now rising from […]

Groundbreaking ceremony for the modern new building.
Chiracon’s ground-breaking ceremony for the construction of a state-of-the-art production facility for APIs made in Luckenwalde.

Finalist of Großer Preis des Mittelstandes
Chiracon is among the five finalists for the Großer Preis des Mittelstands. Nominated by the district of Teltow-Fläming, Office for Economic Promotion and District Development, the innovative, research-based pharmaceutical company prevailed against over 4300 companies in four selection rounds.

Honoured with Zukunftspreis Brandenburg 2022
Chiracon was honoured with the “Zukunftspreis Brandenburg” 2022. With their business ideas and developments, they convinced the high-calibre 16-member jury of their special achievements for the Brandenburg economy.

